Abstract
Background: Persistent or recurrent measurable residual disease (MRD) is associated with high rates of relapse and poor survival in B-cell acute lymphoblastic leukemia (ALL). We aimed to evaluate the anti-CD22 antibody-drug conjugate, inotuzumab ozogamicin (INO), in patients (pts) with B-cell ALL in complete morphologic remission who did not achieve MRD negativity with conventional therapy or who experienced MRD recurrence.
Methods: This is a single-arm, phase II trial of pts with B-cell ALL in complete remission (CR) who did not achieve MRD negativity or had MRD-positive relapse after at least 3 months from the start of frontline therapy (i.e. CR1) or 1 month from the start of any salvage therapy (i.e. CR2 and beyond). Positive MRD was defined as ≥0.01% by multiparameter flow cytometry in pts with Philadelphia chromosome (Ph)-negative ALL and a BCR-ABL1 to ABL1 transcript ratio by PCR of ≥0.01% for pts with Ph-positive ALL. MRD negativity was defined as undetectable MRD by flow and PCR at a sensitivity of at least 10 -4. INO was given at a dose of 0.6 mg/m 2 on day 1 and 0.3 mg/m 2 on day 8 of cycle 1 and 0.3 mg/m 2 on days 1 and 8 of subsequent cycles (up to 6 total cycles, given every 21-28 days). Ursodiol prophylaxis was given to all pts. Subsequent allogeneic stem cell transplantation (ASCT) was offered depending on donor availability and pt fitness. Pts with Ph+ ALL received concomitant therapy with a TKI. The choice of TKI was up to the treating physician.
Results: Between 11/2018 and 5/2021, 16 pts with MRD-positive B-cell ALL in CR were enrolled. The median age was 47 years (range, 20-67 years). Ten pts (63%) had Ph-positive ALL; 9 pts received concomitant ponatinib, and 1 received dasatinib. Eleven pts (69%) were in CR1, and 5 pts (31%) were in CR2 and beyond (3 in CR2 and 2 in CR3). Ten pts (62.5%) had persistent MRD and 6 (37.5%) had MRD recurrence. Nine (56%) pts had received prior blinatumomab, 3 (19%) pts had undergone prior ASCT, and one (6%) patient had undergone CAR-T.
Pts received a median of 3 cycles of INO consolidation (range, 1-6 cycles). Overall, 8 pts (50%) achieved MRD negativity at any time. Four of 6 pts (67%) with Ph-negative ALL achieved MRD negativity by flow cytometry, and 4 of 10 pts (40%) with Ph-positive ALL achieved MRD negativity by PCR. Four additional pts with Ph-positive ALL achieved a major molecular response (MMR) as best response. Among the 8 MRD responders, 7 pts (87.5%) responded after 1 cycle, and 1 (12.5%) responded after 2 cycles. Five of 7 pts (71%) with no prior blinatumomab exposure responded to INO whereas only 3 of 9 pts (33%) with prior blinatumomab responded. Five pts (63% of MRD responders) proceeded to ASCT (1 in CR1 and 4 in CR2+) with median time to ASCT of 4 months (range, 2-5 months).
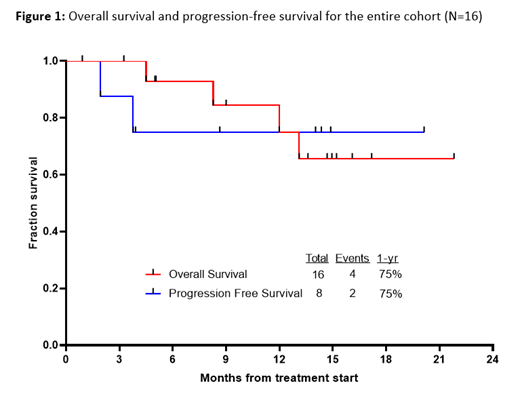
With a median follow-up of 14 months (range, 1-22 months), 1 pt relapsed (13% of MRD responders). The estimated 1-year overall survival (OS) and relapse-free survival (RFS) rates were both 75% (Figure 1). The 1-year OS rates for responders and non-responders were 86% and 63%, respectively. One responding patient died (from post-ASCT complications), and 3 non-responding pts died (2 from disease progression and 1 from unknown causes). Among the 3 responders who did not undergo ASCT, 1 subsequently relapsed, and all are still alive. Among the 5 responders who underwent ASCT, no pts have subsequently relapsed; 1 pt died from post-ASCT complications, and the 1-year OS rate of transplanted pts was 75%.
INO-related adverse events were as expected. The only grade 4 events were hematologic; 6 pts (38%) developed grade 4 neutropenia and 2 pts (13%) developed grade 4 thrombocytopenia. Grade 3 events included: 4 pts (25%) with thrombocytopenia, 1 pt (6%) with anemia, and 2 pts with transaminase elevation. One patient discontinued study treatment due grade 3 veno-occlusive disease after 5 courses of INO.
Conclusion: INO is a well-tolerated and effective agent to eradicate MRD in pts with B-cell ALL who have persistent MRD or who experience MRD recurrence.
Short: Novartis: Honoraria; NGMBio: Consultancy; Jazz Pharmaceuticals: Consultancy; AstraZeneca: Consultancy; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria. Kantarjian: Aptitude Health: Honoraria; NOVA Research: Honoraria; Ipsen Pharmaceuticals: Honoraria; KAHR Medical Ltd: Honoraria; Astellas Health: Honoraria; Pfizer: Honoraria, Research Funding; Astra Zeneca: Honoraria; Immunogen: Research Funding; Daiichi-Sankyo: Research Funding; BMS: Research Funding; Ascentage: Research Funding; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Jazz: Research Funding; Novartis: Honoraria, Research Funding; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Alvarado: MEI Pharma: Research Funding; Jazz Pharmaceuticals: Research Funding; CytomX Therapeutics: Consultancy; Astex Pharmaceuticals: Research Funding; FibroGen: Research Funding; BerGenBio: Research Funding; Sun Pharma: Consultancy, Research Funding; Daiichi-Sankyo: Research Funding. Burger: Pharmacyclics LLC: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Beigene: Research Funding, Speakers Bureau; Gilead: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; TG Therapeutics: Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Novartis: Other: Travel/Accommodations/Expenses, Speakers Bureau; AstraZeneca: Consultancy; Janssen: Consultancy, Other: Travel/Accommodations/Expenses, Speakers Bureau. Jain: Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; Janssen: Honoraria; TG Therapeutics: Honoraria; AstraZeneca: Honoraria, Research Funding; Beigene: Honoraria; Incyte: Research Funding; Genentech: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; Precision Biosciences: Honoraria, Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; Servier: Honoraria, Research Funding; Pfizer: Research Funding; AbbVie: Honoraria, Research Funding; Pharmacyclics: Research Funding. Konopleva: Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Forty Seven: Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Ascentage: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Agios: Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; Cellectis: Other: grant support; KisoJi: Research Funding; Sanofi: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights. Ravandi: Taiho: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astex: Honoraria, Research Funding; Prelude: Research Funding; AstraZeneca: Honoraria; Xencor: Honoraria, Research Funding; Novartis: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Agios: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding. DiNardo: Agios/Servier: Consultancy, Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; Takeda: Honoraria; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; AbbVie: Consultancy, Research Funding; Forma: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Sasaki: Novartis: Consultancy, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees. Thompson: Genentech: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Amgen: Other: Institution: Honoraria, Research Grant/Funding; AbbVie: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Adaptive Biotechnologies: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding, Expert Testimony; Pharmacyclics: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Janssen: Consultancy, Honoraria; Gilead: Other: Institution: Advisory/Consultancy, Honoraria. Ferrajoli: BeiGene: Other: Advisory Board, Research Funding; AstraZeneca: Other: Advisory Board, Research Funding; Janssen: Other: Advisory Board . Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding.
Inotuzumab ozogamicin for MRD+ B-cell ALL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal